 |
Detection of Embolic Signals in Cranial Blood Flow
Joint project with Prof Hugh Markus of St Georges Hospital,
Univesity of London |
Stroke is a major cause of death in the UK and the most common cause of adult neurological disability. In over three quarters of cases stroke is caused by blockage of blood vessels to the brain and a reduction in blood supply (ischaemic stroke). Of these, about a half are caused by emboli, which are blood clots, which form in one part of the vascular system, and then circulate through the blood vessels distally to one of the brain blood vessels, which they block.
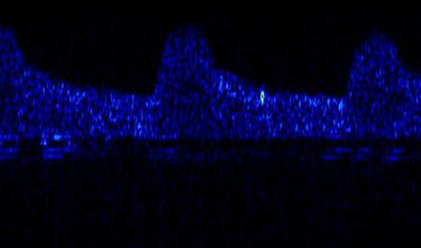
The mainstay of stroke treatment is prevention. Therefore identifying individuals at high risk of stroke who can be treated with appropriate surgical or medical therapies is of great importance. Potential treatments in patients with stroke caused by emboli include carotid endarterectomy to clean out vessels in the neck and remove atheroma (fatty deposits) causing narrowing on which emboli can form, and the use of drugs such as aspirin to reduce the formation of emboli. In the past it has only been possible to tell that somebody has had an embolic stroke indirectly. For example in a patient who has suffered stroke, a potential source of emboli, such as a narrowed artery in the neck, may be found and assumed to be the cause. However, more recently it has been possible to directly measure emboli as they pass through the brain circulation using a technique called Transcranial Doppler Ultrasound (TCD). Emboli are seen as short duration high energy signals in the Doppler spectrum because they reflect more of the ultrasound signal than the surrounding red blood cells. An example is shown above. The vertical axis is velocity the horizontal axis is time and the brightness is energy. The background blood flow is seen in dark blue. Three heart beats can be seen rising to a maximum velocity and then falling away. The embolic signal is the bright spot between the second and third beats.
The detection of solid emboli composed of material such as thrombus (blood clots) and platelets was first described in 1990. Studies in the early 1990’s showed that the technique was highly sensitive and specific. It was then shown that the such asymptomatic circulating embolic signals could be detected in patients with a variety of types of stroke. Most work has been done in patients with carotid artery stenosis; in this condition there is a narrowed artery in the neck which produces a nidus on which platelets and thrombus aggregate and form emboli. In this situation the presence of embolic signals independently predicts future stroke risk. The technique may therefore be a useful marker to stratify patients and identify which ones need particularly aggressive therapies. It has been used recently to evaluate new therapies, both in single centre studies and in a multi-centre international study.
A major problem impairing widespread use of this technique on a clinical basis is a lack of reliable automated systems for embolic signal detection. The current gold standard for detecting emboli is a trained observer. It has been shown in international multi-centre studies that there is a high degree of agreement between different experienced observers. However, the experienced observer needs to review the data in real time. This is costly in human time and therefore infeasible in a clinical context where recordings of at least one hour duration are normally used for each patient. A further drawback of using human observers is that fatigue may reduce the accuracy of long assessments. The problem has become more acute with the recent introduction of ambulatory Transcranial DopplerTCD (pioneered at St George's Hospital) in which recordings are performed for up to 8 hours. Initial attempts at automated embolic signal detection were performed using simple systems that identified a short duration spectral energy increase. The early work in this field which was not particularly successful but now a commercially available system for counting emboli, named FS-1, reports sensitivities of up to 85% for certain conditions. It uses the short time window Fourier transform to extract frequency features from the audio source and classifies them using fuzzy logic. A comprehensive evaluation of FS-1 demonstrated that it is highly effective for patients with symptomatic carotid stenosis and post carotid enarterectomy, but less good and not adequate for clinical use in patients with atrial fibrillation and asymptomatic carotid stenosis in whom embolic signals are less frequent and of lower intensity. In atrial fibrillation its sensitivity was only 54% at a very low specificity of 7%. It is also less accurate in the presence of high background noise.
An alternative approach to the detection of embolic signals is to use a wavelet transform to extract features rather than a short time window Fourier Transform. Marvasti, Gillies, Marvasti and Markus (2004) demonstrated that detection could be achieved in real time using the discrete wavelet transform. This system significantly outperformed FS-1 on small emboli (<7dB above the background). FS-1 still provided more accurate results for larger higher intensity embolic signals, but this could simply be because the feature set extracted from the wavelet transform was not optimal.
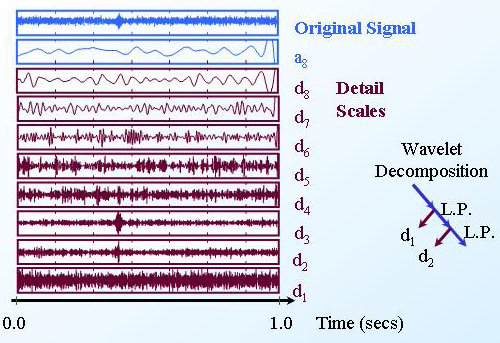 |
Wavelet decomposition of a TCD Ultrasound signal
The wavelet transform can help to identify embolic signals. Here the
embolic signal is seen clearly in detail scales d2 and d3. |